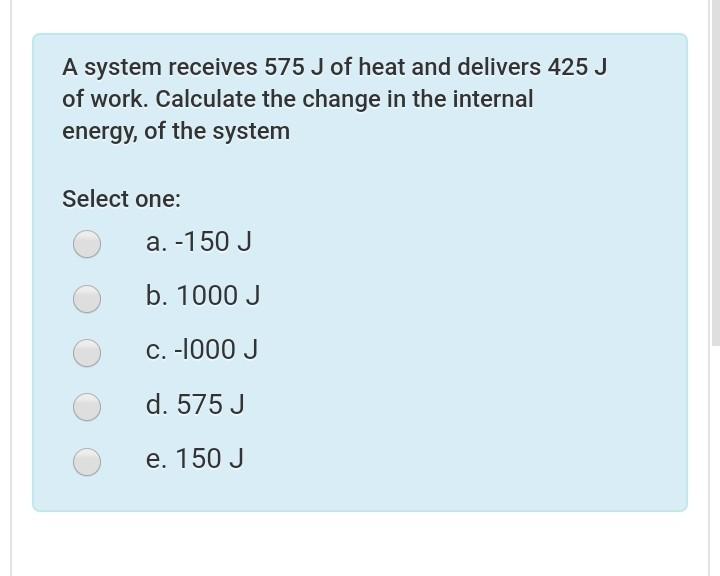
85+ pages a system receives 575 j of heat 5mb. A system receives 575 J of heat and delivers 425 J of work. A system receives 575 J of heat and delivers 425 J of work. Calculate the change in the internal energy D E of the system. Read also solution and understand more manual guide in a system receives 575 j of heat School University of New England.
A system receives 575 J of heat and delivers 425 J of work Calculate the change. Calculate the change in the internal energy of the system Select one.

Thursday Questions 101019 Docx 1 A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change In The Internal Energy E Of The Course Hero
| Title: Thursday Questions 101019 Docx 1 A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change In The Internal Energy E Of The Course Hero |
| Format: PDF |
| Number of Pages: 293 pages A System Receives 575 J Of Heat |
| Publication Date: June 2021 |
| File Size: 1.3mb |
| Read Thursday Questions 101019 Docx 1 A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change In The Internal Energy E Of The Course Hero |
 |
Solution for A system receives 575 J of heat and delivers 425 J of work.

A system receives 575 J of heat and delivers 425 J of work. Delta E of the system. A system delivers 225 J of heat to the surroundings while delivering 645 J of work. A system receives 575 J of heat and delivers 425 J of work. Calculate the change in the internal energy. Calculate the change in the internal energy of the system Select one.

Thursday Questions 101019 Docx 1 A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change In The Internal Energy E Of The Course Hero
| Title: Thursday Questions 101019 Docx 1 A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change In The Internal Energy E Of The Course Hero |
| Format: PDF |
| Number of Pages: 199 pages A System Receives 575 J Of Heat |
| Publication Date: January 2021 |
| File Size: 6mb |
| Read Thursday Questions 101019 Docx 1 A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change In The Internal Energy E Of The Course Hero |
 |

A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change Course Hero
| Title: A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change Course Hero |
| Format: PDF |
| Number of Pages: 213 pages A System Receives 575 J Of Heat |
| Publication Date: October 2018 |
| File Size: 800kb |
| Read A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change Course Hero |
 |
A System Receives 575 J Of Heat And Delivers 425 J Of Chegg
| Title: A System Receives 575 J Of Heat And Delivers 425 J Of Chegg |
| Format: PDF |
| Number of Pages: 187 pages A System Receives 575 J Of Heat |
| Publication Date: May 2020 |
| File Size: 725kb |
| Read A System Receives 575 J Of Heat And Delivers 425 J Of Chegg |
 |

What Is The Change In Internal Energy In J Of A System That Absorbs 0 464 Kj Of Heat From Its Surroundings And Has 0 630 Kcal Of Work Done On It Socratic
| Title: What Is The Change In Internal Energy In J Of A System That Absorbs 0 464 Kj Of Heat From Its Surroundings And Has 0 630 Kcal Of Work Done On It Socratic |
| Format: PDF |
| Number of Pages: 266 pages A System Receives 575 J Of Heat |
| Publication Date: October 2017 |
| File Size: 1.6mb |
| Read What Is The Change In Internal Energy In J Of A System That Absorbs 0 464 Kj Of Heat From Its Surroundings And Has 0 630 Kcal Of Work Done On It Socratic |
 |
When Ammonium Chloride Crystals Are Dissolved In Chegg
| Title: When Ammonium Chloride Crystals Are Dissolved In Chegg |
| Format: ePub Book |
| Number of Pages: 208 pages A System Receives 575 J Of Heat |
| Publication Date: July 2018 |
| File Size: 1.7mb |
| Read When Ammonium Chloride Crystals Are Dissolved In Chegg |
 |

Answer What Is The Change In The Internal Clutch Prep
| Title: Answer What Is The Change In The Internal Clutch Prep |
| Format: ePub Book |
| Number of Pages: 312 pages A System Receives 575 J Of Heat |
| Publication Date: June 2021 |
| File Size: 2.6mb |
| Read Answer What Is The Change In The Internal Clutch Prep |
 |

A System Receives 425 J Of Heat From And D Clutch Prep
| Title: A System Receives 425 J Of Heat From And D Clutch Prep |
| Format: ePub Book |
| Number of Pages: 248 pages A System Receives 575 J Of Heat |
| Publication Date: July 2019 |
| File Size: 6mb |
| Read A System Receives 425 J Of Heat From And D Clutch Prep |
 |

1 A System Receives 575 J Of Heat And Delivers 425 J Chegg
| Title: 1 A System Receives 575 J Of Heat And Delivers 425 J Chegg |
| Format: eBook |
| Number of Pages: 255 pages A System Receives 575 J Of Heat |
| Publication Date: February 2017 |
| File Size: 800kb |
| Read 1 A System Receives 575 J Of Heat And Delivers 425 J Chegg |
 |
A System Receives 575 J Of Heat And Delivers 425 J Of Chegg
| Title: A System Receives 575 J Of Heat And Delivers 425 J Of Chegg |
| Format: ePub Book |
| Number of Pages: 336 pages A System Receives 575 J Of Heat |
| Publication Date: December 2021 |
| File Size: 1.9mb |
| Read A System Receives 575 J Of Heat And Delivers 425 J Of Chegg |
 |
Receives 575 J Of Head And Delivers 425 J Of Work Chegg
| Title: Receives 575 J Of Head And Delivers 425 J Of Work Chegg |
| Format: PDF |
| Number of Pages: 206 pages A System Receives 575 J Of Heat |
| Publication Date: May 2021 |
| File Size: 1.7mb |
| Read Receives 575 J Of Head And Delivers 425 J Of Work Chegg |
 |

A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change Course Hero
| Title: A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change Course Hero |
| Format: PDF |
| Number of Pages: 191 pages A System Receives 575 J Of Heat |
| Publication Date: October 2017 |
| File Size: 1.4mb |
| Read A System Receives 575 J Of Heat And Delivers 425 J Of Work Calculate The Change Course Hero |
 |
In the dynamic world we currently live in its becoming increasingly difficult for students to balance academics co-curricular activities. A system receives 575 J of heat and delivers 425 J of work. A system receives 575 J of heat and delivers 425 J of work.
Here is all you have to to learn about a system receives 575 j of heat Time min c4h6 m 0 0. Calculate the change in the internal energy. A 150 J b 150 J c l000 J d 1000 J e 575 J. Answer what is the change in the internal clutch prep a system receives 425 j of heat from and d clutch prep what is the change in internal energy in j of a system that absorbs 0 464 kj of heat from its surroundings and has 0 630 kcal of work done on it socratic thursday questions 101019 docx 1 a system receives 575 j of heat and delivers 425 j of work calculate the change in the internal energy e of the course hero when ammonium chloride crystals are dissolved in chegg a system receives 575 j of heat and delivers 425 j of work calculate the change course hero A system receives 575 J of heat and delivers 425 J of work.







FOLLOW THE Zoey Books Chapter AT TWITTER TO GET THE LATEST INFORMATION OR UPDATE
Follow Zoey Books Chapter on Instagram to get the latest information or updates
Follow our Instagram